| Deutsch | English |  |
||
| Nasal
breathing Nasal stents Sleep apnea Testimonials Service/Ordering Imprint Disclaimer Data protection |
Sleep apnea and nasal stents
|
|||
|
The cause of sleep apnea typically is said to be a
falling back of the tongue. However, according to
scientific literature, this only applies to a small
proportion of patients. In most patients, the soft
palate and upper pharyngeal walls suck togeth. The
causes of these obstructions are physical and can be
anatomical or due to swelling in the nose, as well as
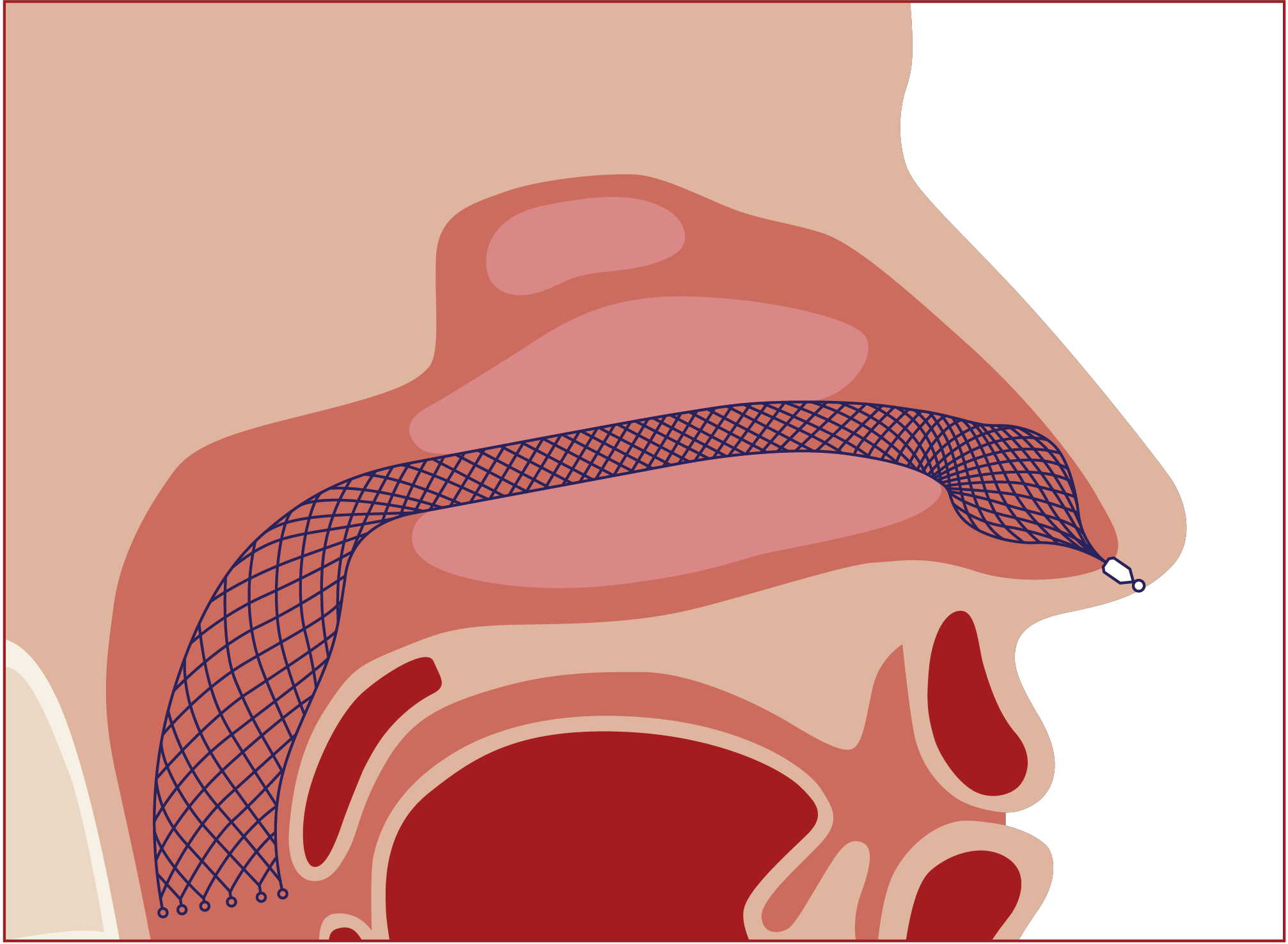
due to impaired nasal function. - the AlaxoLito Plus or Xtreme Nasal Stents to improve nasal breathing (see "nasal breathing") - for CPAP users to prevent nasal alar compression as well as to counteract the frequent swelling of the nasal turbinates under artificial ventilation and to keep the CPAP pressure lower. The Alaxo Hybrid Stent is a new development that combines 15 years of experience with nasal stents in daily use, including from numerous clinical investigations on sleep apnea patients, as well as from intensive scientific analyses and evaluations in an optimized product concept. 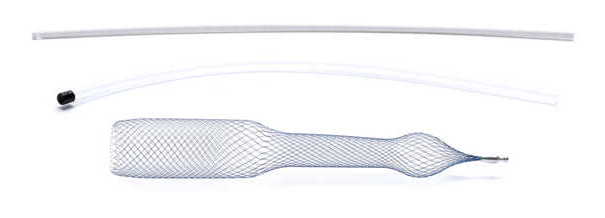 Continuous splinting of the nasal passage and nasopharynx in the region of the soft palate optimizes the airway in all critical regions. This improves nasal breathing and prevents the symptom of airway obstruction at the soft palate where the symptom occurs. The Alaxo Hybrid Stent is an AlaxoLito Plus nasal stent extended by an additional section that splints the soft palate. The Alaxo Hybrid Stent is inserted into one nasal passage up to the soft palate and positioned with the ball-shaped part in the nostril so that application is easy.
An AlaxoLito Plus nasal stent is inserted into the second nasal passage (see "nasal breathing").
|
|||